EURL rabies
Collection of samples, techniques, validation and interpretation of the diagnostic methods for the purposes of rabies surveillance are presented here.
Diagnostic methods considered compliant are presented in the WOAH rabies manual. A list of procedures inspired by the WOAH official methods is also proposed.
Lorem ipsum dolor sit amet, consectetuer adipiscing elit. Maecenas porttitor congue massa. Fusce posuere, magna sed pulvinar ultricies, purus lectus malesuada libero, sit amet commodo magna eros quis urna.
Nunc viverra imperdiet enim. Fusce est. Vivamus a tellus.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Proin pharetra nonummy pede. Mauris et orci.
Aenean nec lorem. In porttitor. Donec laoreet nonummy augue.
Suspendisse dui purus, scelerisque at, vulputate vitae, pretium mattis, nunc. Mauris eget neque at sem venenatis eleifend. Ut nonummy.
Reference collection
Last update: 29/07/2025
Rabies disease
Rabies is a fatal zoonotic disease (animal disease that can be transmitted to humans) caused by a virus of the genus Lyssavirus. Excreted in the saliva of infected mammals in the final phases of the disease, the virus is generally transmitted to another animal or to humans through biting. Contamination may also occur if the saliva of an infected animal comes into contact with an open wound or a mucous membrane. Without post-exposure treatment prior to the onset of clinical signs, the disease is invariably fatal.
Rabies, which causes over 59,000 human deaths a year worldwide, is found all over the world, except in certain areas such as Antarctica. Several European countries have become rabies-free in non flying mammals thanks to oral vaccination programmes of wildlife.
There are 16 different rabies virus species, seven of which transmission to human has already been notified. Those species are mainly differentiated according to the animal host species. Rabies due to rabies virus species (RABV) is responsible for most human and animal rabies cases.
In industrialised countries, rabies persists mainly in wild animals, whereas in many developing countries it remains an endemic disease, with the domestic dog as principal reservoir and main source of human contamination.
In European countries, rabies in dogs was eliminated several decades ago, but it continued to persist and spread in fox and racoon dog populations. Thanks to oral vaccination campaigns conducted in wildlife, the incidence of rabies in both domestic and wild animals in the EU has drastically reduced. Rabies has been eliminated from the Netherlands, Finland, Switzerland, France, Belgium, Luxembourg, Czech Republic, Austria, Germany, Estonia, Italy, Latvia and Slovenia.The elimination of rabies in non-flying mamals (RABV) in the European Union will be reachable in the next years. In 2018 and 2019, eight (from 3 EU countries) and five (from 2 EU countries) cases, respectively, were reported in the EU.
To detect timely any suspect animal, the rabies situation in all Europe should be continuously monitored, based on surveillance programmes. The illegal importation of infected cats and dogs from endemic countries remains a major concern, with regular rabies alerts occuring (ProMED). In Europe, bat rabies cases are attributed to five different lyssavirus species. While European bat lyssavirus types 1 and 2 are responsible for most bat rabies cases, Bokeloh bat lyssavirus, West Caucasian bat lyssavirus and Lleida bat lyssavirus have occasionnaly been isolated.
Additional documentation
...
EU National Reference Laboratories for rabies
List of the EU National Reference Laboratories for rabies, including presentation (for private members only) and official website:
AUSTRIA
Presentation: Austrian NRL
Official website: Austrian Agency for Health and Food Safety (AGES), Institute for Veterinary Disease Control
BELGIUM
Presentation: Belgian NRL
Official website: Sciensano
BULGARIA
Presentation: Bulgarian NRL
Laboratory: Bulgarian Food Safety Agency (BFSA ) National Diagnostic Research Veterinary Institute (NDRVI)
CROATIA (Republic of)
Presentation: Croatian NRL
Official website: Croatian Veterinary Institute
CYPRUS
Presentation: Cypriot NRL
Official website: Animal Health Laboratory - Virology section
CZECH REPUBLIC
Presentation: Czech NRL
Official website: State Veterinary Institute Prague
DENMARK
Presentation: Danish NRL
Official website: DTU Vet
ESTONIA
Presentation: Estonian NRL
Official website: Estonian veterinary and Food laboratory
FINLAND
Presentation: Finnish NRL
Official website: Finnish Food Safety Authority Evira
FRANCE
Presentation: French NRL and EURL
Official website: Anses-Nancy Laboratory for rabies and wildlife
GERMANY
Presentation: German NRL
Official website: Friedrich-Loeffler-Institut - Federal Research Institute for Animal Health
GREECE
Presentation: Greek NRL
Laboratory: Virology Laboratory, Department of Molecular Diagnostics, FMD, Virological, Ricketsial and Exotic Diseases, Athens Veterinary Center
HUNGARY
Presentation: Hungarian NRL (sorry, no file)
Official website: Veterinary Diagnostic Directorate, National Food Chain Safety Office
IRELAND
Presentation: Irish NRL
Official website: Central Veterinary Research Laboratory (CVRL), Department of Agriculture, Food and the Marine
ITALY
Presentation: Italian NRL
Official website: Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe)
LATVIA
Presentation: Latvian NRL
Official website: Institute of Food Safety, Animal Health and Environment BIOR
LITHUANIA
Presentation: Lithuanian NRL
Official website: National Food and Veterinary Risk Assessment Institute
THE NETHERLAND
Presentation: Dutch NRL
Official website: Central Veterinary Institute of Wageningen UR
POLAND
Presentation: Polish NRL
Official website: National Veterinary Research Institute - Department of Virology
PORTUGAL
Presentation: Portuguese NRL (sorry, no file)
Official website: INIAV, Instituto Nacional de Investigação Agrária e Veterinária, I.P. (In Portuguese)
ROMANIA
Presentation: Romanian NRL
Official website: Institute for Diagnosis and Animal Health Bucharest - Virology Department (in Romanian)
SLOVAKIA
Presentation: Slovak NRL (sorry, no file)
Official website: State Veterinary and Food Institute
SLOVENIA
Presentation: Slovenian NRL
Official website: National veterinary institute
SPAIN
Presentation: Spanish NRL
Official website: Instituto de Salud Carlos III, Centro Nacional de Microbiología
SPAIN
Official website: Laboratorio Central de Sanidad Animal (Santa Fe-Granada) (LCSA) (mapa.gob.es)
SWEDEN
Presentation: Swedish NRL
Official website: SVA, National Veterinary Institute
Last update: 14 january 2022
Former Workshops documents
Search a document
Duties of the European Union Reference Laboratory for Rabies
Coordinating the methods employed in the Member States for diagnosing rabies
● Typing, storing and supplying strains of rabies virus
● Preparing, controlling and supplying international standard sera and other reference reagents to the National Reference Laboratories in order to standardise the tests and reagents used in the Member States
● Validating reference reagents including antigens and national standard sera submitted by the National Reference Laboratories
● Building up and maintaining a sera bank and a collection of rabies virus, and maintaining a database of strains isolated across the European Union, including typing
● Organising periodical comparative tests of diagnostic procedures at European Union level and operating laboratory proficiency tests of National Reference Laboratories
● Collecting and collating data and information on the methods of diagnosis used and the results of tests carried out in the European Union
● Characterising rabies virus by the most up-to-date methods available to allow a greater understanding of the epidemiology of the disease
● Keeping abreast of developments in rabies surveillance, epidemiology and prevention throughout the world
● Acquiring a thorough knowledge of the preparation and use of the products of veterinary immunology used to eradicate and control rabies, including the evaluation of vaccines
Contributing to the improvement and harmonisation of methods of analysis throughout the European Union
● By specifying standard test methodologies
● By providing reference materials to NRLs
● By providing NRLs with details and guidance on the methods of laboratory analysis, testing or diagnosis
● By conducting training courses for staff from NRLs, and if needed from other official laboratories
Organising workshops for the benefit of National Reference Laboratories
● Including training of experts from the Member States and, as appropriate, from third countries, in new analytical methodologies
Providing scientific and technical assistance to the Commission
● And, upon its request, by participating in international forums relating to rabies, concerning in particular the standardisation of analytical diagnostic methods and their implementation
Performing and coordinating research activities directed towards the improved control and eradication of rabies
● Carrying out or collaborating with National Reference Laboratories in test validation trials
● Providing scientific advice to the Commission and collecting information and reports associated with the activities of the European Union Reference Laboratory
MAIN PARTNERS
European Commission www.ec.europa.eu
WOAH - World Organisation for Animal Health www.woah.org/en/home/
WHO - World Health Organisation www.who.int
EFSA - European Food Safety Authority www.efsa.europa.eu
EDQM - European Directorate for the Quality of Medicines and Healthcare www.edqm.eu
FAO - Food and Agriculture Organisation www.fao.org
GARC - Global Alliance for Rabies Control https://rabiesalliance.org/
ADDITIONAL DOCUMENTATION
...
European Commission website and regulation
- Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 (Repeal of Regulation (EC) No 882/2004)
- Commission Regulation (EC) No 415/2013 of 6 May 2013 (Amendment of Regulation (EC) No 737/2008)
- Regulation (EC) No 208/2011 (Amendment of Annex VII to Regulation (EC) No 882/2004)
- Commission Regulation (EC) No 737/2008 of 28 July 2008 (Nomination of Anses as EURL for rabies)
- Regulation (EC) No 882/2004 (Official controls)
- List of European Union Reference Laboratories
Last update : 17 January 2023
Other international activities
Last update: 17 January 2020
The laboratory is:
- WHO Collaborating Centre for Research and Management in zoonosis control since September 1983,
- WOAH Reference Laboratory for rabies since June 1991,
- European Union Reference Laboratory for rabies serology since March 2000,
- European Union Reference Laboratory for rabies since July 2008.
- Official Medicines Control Laboratory (for inactivated and oral rabies vaccines) since 2012.
The laboratory provides scientific and technical support at the European level (European Commission, EDQM, EFSA), and to international organizations (OIE, WHO) through numerous activities. The laboratory works in close cooperation with the National Reference Laboratories and their competent authorities for helping to define the strategy and the organization of oral vaccination programmes, as well as for establishing the surveillance and the monitoring of the disease (incidence of rabies, assessment of bait uptake and of rabies immunity both in adult and young target animals).
The laboratory ensures training activities in the frame of its own programmes, and collaborates in the preparation and distribution of teaching materials.
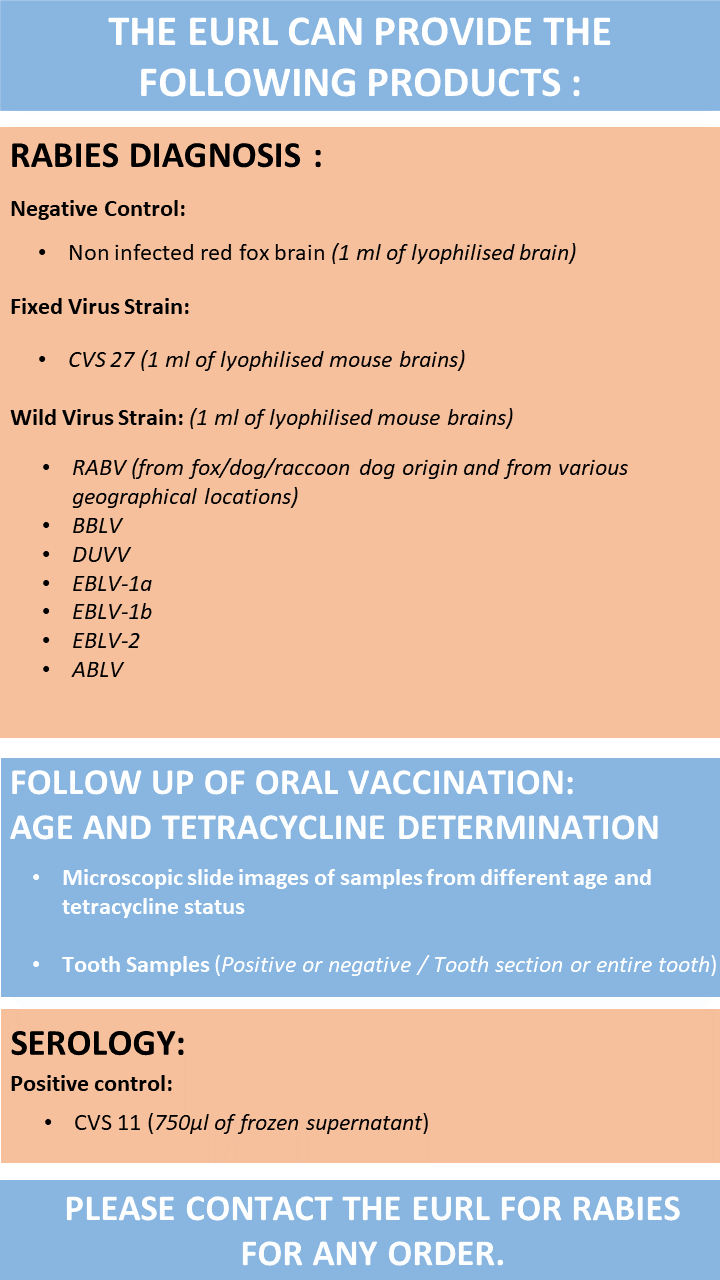
Biological reference material and laboratory services are available on request to help international laboratories to develop their own rabies surveillance and monitoring systems, as well as any other laboratory services for rabies research and epidemiology.
The rabies laboratory's experts provide scientific and technical assistance, advice and training for Member States and third countries. They are also involved in evaluation studies for improving and adapting existing diagnostic, serological, molecular biology and epidemiological tools.
..
Additional documentation
..
Links
A non-exhaustive list of main partners of the network:
EURL technical evaluation
Search a document
How to contact us?
Postal address:
FRENCH AGENCY FOR FOOD, ENVIRONMENTAL AND OCCUPATIONAL HEALTH & SAFETY
Nancy Laboratory for Rabies and Wildlife
Batiment H
Technopôle Agricole et Vétérinaire
CS 40 009
54220 Malzéville - France
Phone: +33 (0)3 83 29 89 50
E-Mail: For any questions or remarks, please contact us by email.